LIGHT
WHAT IS LIGHT?
Answering this question is not as simple as it would seem. Colloquially, we use the word ‘light’ to refer to the luminous, often colorful, medium that allows us to see things and without which we would be cast in darkness. In a more scientific context, light is something called electromagnetic radiation. It is energy that travels through space in the form of oscillating waves of intertwined electric and magnetic fields. Or, wait… isn’t light a bunch of tiny energetic particles moving in a straight line? It’s both? Or neither? Okayyyy. Anyway, this radiation reflects off objects in the environment around us. But sometimes it doesn't… Sometimes it goes right through or bends or splits apart. That’s kinda weird. Eventually, light is intercepted by our eyes and interpreted by our brain as vision. Unless it’s invisible light… Alright, now I'm just confused.
Fear not! We will figure this out. While light is very enigmatic and not explicitly an astronomical concept, it does play possibly the most fundamental role in how we perceive and understand celestial objects and the cosmos through which they flow.
HISTORY OF DISCOVERY
Many ideas have been proposed throughout history to explain what light really is. Some of the earliest ideas were kind of out there but through clever experimentation and engineering, mathematicians and scientists across the world brought exponentially more sophisticated understandings.
One of the first prominent theories came from the ancient Greek philosopher Empedocles in 450 B.C. who suggested that light was actually projected from our own eyes via a “visual fire” placed within them by Aphrodite. This would much later be called emission theory. Despite being pretty off the mark, this idea held sway for centuries by other influential thinkers like Ptolemy and Euclid.
The Golden Age of Islam (7th-13th century) saw many advancements in the field of optics. In the 11th century, renowned Muslim physicist and mathematician Ibn al-Haytham disproved emission theory. In his highly influential “Book of Optics,” he accurately proposed that light reflects off objects, enters the eye where it is focused by a lens and then processed by the brain.
In the late 17th century, a debate was brewing in the scientific community about the nature of light. On one side, Isaac Newton in his book “Opticks” proposed that light is made up of tiny particles. This was the more popular theory at the time. On the other side, Christiaan Huygens authored “Treatise on Light” and suggested in opposition to Newton that light was actually made up of waves. It would later turn out that they were kind of both right. More on that later.
In the early 19th century, scientists like Thomas Young would confirm the wave-like nature of light in his famous double slit experiment. In the 1860’s, James Clerk Maxwell developed the idea that light moved as coupled waves of electricity and magnetism. He also accurately determined the speed of light.
In the early 20th century, Max Planck and later with help from Niels Bohr and Albert Einstein, developed a quantum theory of light that reconciled wave theory with particle theory by establishing a wave-particle duality. Here, light is considered to be both simultaneously. This theory currently represents our best understanding of light.
WHERE DOES LIGHT COME FROM?
Electromagnetic radiation can be produced by a variety of natural and artificial sources and each of those sources can produce different wavelengths. There are two main mechanisms for the generation of electromagnetic radiation: incandescence and luminescence.
INCANDESCENCE
Light can be emitted by objects due to them being very hot. This is called incandescence, thermal radiation or “hot light.”
When you heat up matter, it gains energy which it will then try to release. One of the ways this energy is released is in the form of light. The energy of the light that is emitted depends on how hot it is. The higher the temperature, the higher the energy. Even humans and other animals regularly emit energy in the form of infrared, also known as body heat, which is invisible to our eyes, but we can see it using infrared sensors.
If an object gets hot enough, particularly a dense, solid object, it will begin to glow red in the visible light range. If it gets even hotter (more energetic) it will glow orange or yellow. This is also the main reason why stars are different colors. The coolest stars glow red and orange, intermediate stars glow yellow and white, while the hottest stars glow white and blue.
There is a theoretical object called a black body that perfectly absorbs all light that contacts it and appears completely black. It emits radiation of a specific intensity and color based only on its temperature. Therefore, radiation emitted due to an object’s temperature is referred to as black body radiation.
Some examples of incandescence would be:
🔅High-temperature rock/metal.
Tungsten filament inside an incandescent bulb
Nichrome coils on an electric stove
Molten iron of a forge
Obsidian, pumice and molten rocks (lava) from volcanic eruptions
🔅Friction and pressure.
Meteors and spaceships entering Earth’s atmosphere at high speed
The interiors of planets and other massive sub-stellar objects
🔅Chemical combustion.
Burning of wood and fossil fuels (campfires, gas stoves, furnaces, car engines, coal-fired power plant)
Explosion of gunpowder (guns, fireworks)
🔅Nuclear fusion.
Cores of stars
Nuclear reactors
LUMINESCENCE
Light can also be emitted by objects through non-heat-related processes. This is called luminescence or “cold light.”
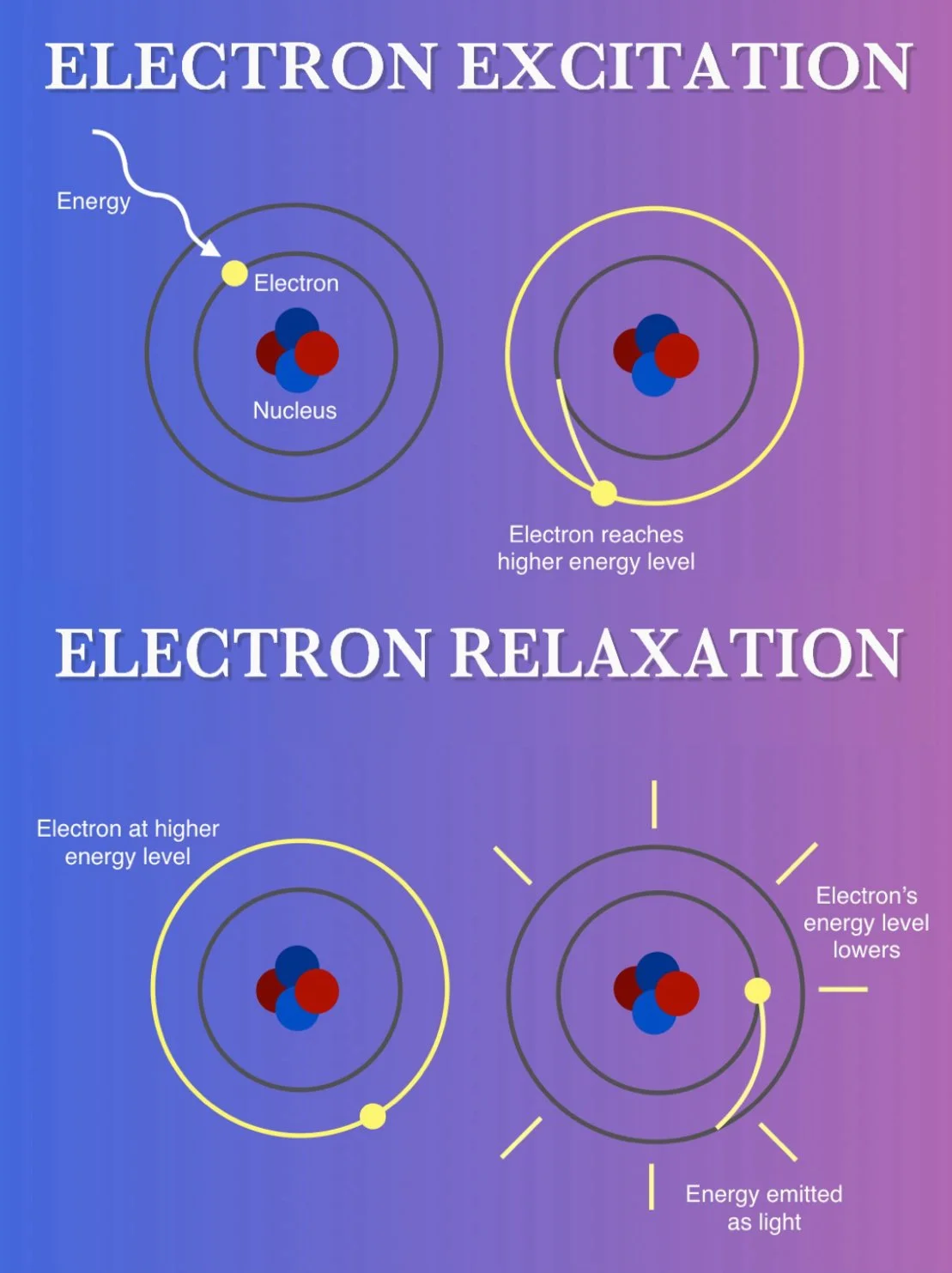
At the center of an atom is a nucleus made up of protons and neutrons. Floating around the nucleus are electrons. Due to incredibly complicated reasons involving quantum mechanics, electrons are only able to occupy a very specific region of space around the nucleus which is determined by the electrons’ energy level.
Imagine the energy level like a set of stairs in which the electron can occupy one step or another but nothing in-between. The more energy the electron absorbs, the higher up the stairs it can go. If the electron can’t absorb enough energy to get to the next step, it doesn’t move. When an electron is excited, it will eventually relax, release the energy and drop down to a lower step. The energy is released by emitting light, just like with incandescent light, but is done without the need for heat.
Some examples of luminescence would be:
🔅Electroluminescence
An electric current is passed through certain kinds of matter.
Semiconductors of light-emitting diodes (television screens, computer monitors)
🔅Chemiluminescence
A reaction caused by the mixing of specific chemicals.
Glow sticks - hydrogen peroxide mixed with fluorescent dye
Forensics - looking for blood at crime scenes by mixing luminol and hydrogen peroxide with the iron in hemoglobin
🔅Bioluminescence
A form of chemiluminescence found in biological organisms.
Insects (fireflies)
Worms (glow worms)
Fish (angler fish)
Fungi (panellus stipticus)
Bacteria (noctiluca scintillans)
🔅Photoluminescence
The absorption of a particular wavelength of light (usually ultraviolet).
Fluorescent lights and paints which relax quickly (last briefly)
Phosphorescent lights and paints which relax gradually (last longer)
🔅Ionization
The addition, but more often removal, of an electron from an atom causes the other electrons to shift around energy levels and release energy.
Lightning is caused by a rapid and intense electrostatic discharge in the atmosphere that both heats and ionizes the air.
Aurorae at the poles of planets are caused by solar wind riding Earth’s magnetic field down and ionizing the atmosphere.
Emission nebulae are ionized by the intense ultraviolet radiation from nearby stars.
THE ELECTROMAGNETIC SPECTRUM
One of the most profound things about light is that all the light we can see with our eyes is but a tiny fraction of all the light there is. There’s an entire universe invisible to us, unless we know how to look for it. This is all part of the electromagnetic spectrum, a broad range of light wavelengths.
What is a wavelength?
Light is generally understood to move in a wave-like manner. These waves oscillate, forming peaks or crests in regular intervals. The wavelength of light is the distance between two consecutive peaks. If there is less distance, the wavelength is said to be shorter with higher energy. If there is more distance, the wavelength is said to be longer with lower energy. It is usually measured in nanometers (nm) or micrometers (μm). The wavelengths of light that our eyes can see directly are called visible light, but the electromagnetic spectrum goes well beyond that.
The EM spectrum ranges are as follows:
Radio waves
Microwaves
Infrared
Visible light
Ultraviolet
X-rays
Gamma rays
These different ranges don’t really have distinct boundaries as is sometimes portrayed but is more of a guideline. Each type has its own effects and applications which humans can take advantage of. Let’s start with the longer, low-energy wavelengths and work our way from there:
RADIO WAVES
Radio waves have the longest wavelengths in the electromagnetic spectrum. This means they have the lowest energy and thus are the least dangerous. Radio waves can be generated by natural sources, such as lightning or astronomical objects like stars and planets.
Applications:
🔅Broadcasting and communications
Data is encoded into the radio waves via amplitude modulation (AM) or frequency modulation (FM), among other methods
Better for wide range
Can move with Earth’s curvature
🔅Astronomical observation
Sun, planets, pulsars, quasars, radio galaxies, cosmic microwave background radiation
Pictured is the Parkes Observatory’s 64-meter radio telescope which was used to receive live footage from the Apollo 11 crew on the Moon.
MICROWAVES
Microwaves have the second longest wavelengths in the electromagnetic spectrum. They used to be classified as just a higher energy, shorter wavelength subset of radio waves which is why they are called microwaves. Today they are recognized as a distinct type of their own.
Stars are natural sources of microwaves but more importantly, scientists used the detection of microwaves to discover the cosmic microwave background radiation which pervades virtually all of the cosmos. This is believed to be, in layman’s terms, the remnant glow from the Big Bang that gave birth to the universe as it exists today.
Applications:
🔅Broadcasting and communications
Better for point to point
Can better penetrate clouds and trees
🔅Cooking
Microwave ovens excite and heat water molecules in food
🔅Doppler radar
Weather forecasting
Speed limit enforcement
🔅Astronomical observation
Cosmic microwave background radiation
INFRARED
Infrared waves lie just outside the lower end of the visible light range. They are often referred to as “heat waves” or “thermal waves” because they act as an effective medium in which heat can be transferred from one object to another. A prime example of this is the heat we feel from the Sun which reaches us via infrared radiation. Your body also gives off infrared. Pretty much anything with a non-zero temperature does.
It was British astronomer William Herschel who first discovered infrared. He used a prism to disperse the different colors of white light on to a surface where he placed a thermometer into each color as well as a control thermometer off to the side to measure room temperature. The temperature of colors increased from violet to red but what he found next was completely unexpected. The control thermometer just to the side of the red spiked much higher in temperature than the others. He discerned that there must be something beyond the visible colors and declared the first instance of what he called “light unfit for vision.”
If violet and blue are higher energy, why did they exhibit lower temperatures in Herschel’s experiment? Herschel failed to account for refraction. When light is refracted, the higher energy wavelengths are bent more and are thus spread out across a surface over a larger area than the lower energy wavelengths. Energy spread out over a larger surface area will exhibit lower temperatures. More on refraction later.
Applications:
🔅Heating
🔅Cooking
Infrared grills and ovens
🔅Security sensors
🔅Night vision
🔅Meteorology
Monitor temperature
🔅Remote control
🔅Fiber optics
🔅Astronomical observation
James Webb Space Telescope: can see protostars through gas and dust clouds, very distant redshifted galaxies
VISIBLE LIGHT
This incredibly narrow band of electromagnetic radiation is the only kind that is visible to the human eye. The biggest source of natural light is that big, bright sphere in the sky that we see everyday, the Sun.
Within the visible light range, it can be further broken down into distinct colors: with red having the longest wavelengths, then going to orange, yellow, green, blue, indigo, and finally violet with the shortest wavelength. Each color has its own narrow range of frequencies. When you see an object that appears a particular color, you are seeing that object reflecting that color, or a combination of colors, at you while absorbing everything else. When all colors are seen at once, we perceive them as white light. However, we can separate these various colors by passing white light through a prism or just go out after a storm and look at a rainbow (we’ll talk later about what causes these). What colors an object reflects and absorbs is based largely on the chemical elements on the surface that the light is interacting with.
To be clear, light beyond the visible range is still there, whether we can see it or not. Let’s use sound as a comparison: There are pitches of sound you can hear, some higher and some lower, but once you start getting to the extreme ends of that, it starts to become inaudible. The vast majority of light that exists is outside the visible range. If the entire electromagnetic spectrum spanned the distance between New York and Los Angeles, the visible light spectrum would be about one inch long.
Applications:
🔅Lighting
Lightbulbs
🔅Electronic displays
Televisions, computer monitors, billboards
🔅Photography and video recording
Cameras
🔅Paint
Different colors (see spectroscopy)
🔅Astronomical observation
Hubble Space Telescope: stars, nebulae, galaxies
ULTRAVIOLET
Ultraviolet lies just outside the higher end of the visible light range (which is just regular violet). They are sometimes referred to simply as UV rays. Although humans can’t see them, there are certain species of insects that can, like bumblebees! These are shorter wavelengths which means higher energy.
The greatest natural source of ultraviolet is sunlight. Ultraviolet is commonly subdivided into three types: UV-A, UV-B and UV-C, all of which are emitted by the Sun. UV-A is the least harmful but prolonged exposure can cause increased skin aging and damage. UV-B rays are a more harmful form that cause sunburns and raise your risk of cancer. UV-C rays are the most harmful, but luckily, the ozone layer in Earth's upper atmosphere absorbs ultraviolet radiation and prevents most of it from reaching Earth.
High energy ultraviolet light also interacts with Earth’s magnetic poles, exciting the atoms and causes a beautiful display of light called an aurora. The color of the aurora can tell you what kind of atom was excited. For example, green light indicates low-altitude oxygen. Red light indicates high-altitude oxygen or nitrogen. Aurorae are found at the poles of other planets as well, like Jupiter and Saturn.
Applications:
🔅Tanning beds
Triggers melanin production in the skin
🔅Sterilization of medical equipment
🔅Skin cancer treatments
🔅Curing inks, varnishes and adhesives
🔅Astronomical observation
Heavier, hotter stars emit more ultraviolet light
X-RAYS
X-rays are very high-frequency waves, meaning short. So short, in fact, that some x-ray waves are no longer than an atom. This means that they carry a significant amount of energy. Natural sources of x-rays are radon gas, radioactive elements in the Earth and cosmic rays that hit the Earth from outer space.
Applications:
🔅Medical and dental diagnostics
Detect bone fractures and tumors
🔅Security screenings
Airports
High-profile events
🔅Electronic component inspection
🔅Geological surveys
🔅Astronomical observation
Chandra X-ray Observatory: supernova remnants, black holes, neutron stars, map the distribution of dark matter
GAMMA RAYS
Gamma rays have the shortest wavelengths and are the most dangerous form of electromagnetic radiation. They are produced by the hottest and most energetic objects in the universe, such as neutron stars (especially pulsars), supernova explosions, and regions around black holes. Gamma-ray wavelengths are so short that they can pass through the space within the atoms of a detector and thus can’t even be intercepted and reflected by mirrors. They just pass right on through. That is why we require special devices to detect them. Gamma-ray detectors contain densely packed crystal blocks. The electrons in the crystals collide with passing gamma-rays in a process called Compton scattering. These collisions create charged particles that can be detected by the sensor.
Gamma-ray bursts (GRB) are the most energetic and luminous electromagnetic events in the known universe, excluding the Big Bang. These terrifying events are believed to be caused by powerful supernovae that result in the creation of black holes. A gamma-ray burst hitting Earth could be catastrophic, obliterating the ozone layer in our upper atmosphere and exposing us to dangerous UV radiation from the Sun. Rest assured, this is incredibly unlikely to ever happen. They are detected across the universe every day, but they are too far away to cause any concern and there are no stars within 200 light years of Earth that are likely to end this way. We should continue to study them just in case we can develop a proper defense against them.
Applications:
🔅Sterilization and disinfection
🔅Food preservation
🔅Medical diagnostics
🔅Cancer treatments
🔅Ground surveys
Finding minerals or fossil fuels
Determining integrity of construction sites
🔅Material inspection
Manufacturing: detect cracks and weaknesses
🔅Astronomical observation
Fermi Gamma-ray Space Telescope: gamma ray bursts, active galactic nuclei, pulsars, supernova remnants, x-ray binary systems
WAVE-PARTICLE DUALITY
As was mentioned early on about the history of our understanding of light, there was a longstanding debate about the nature of light. Scientists like Christiaan Huygens proposed that light was a wave while other scientists like Isaac Newton advocated that light was a particle. So which is it? Is light a wave or a particle? Well, as scientists would come to understand across the 19th and 20th centuries, it’s kind of both yet neither. Light is able to exhibit both wave-like behavior and particle-like behavior depending on how they are observed. This seems incredibly strange as classical particles and waves are structured and behave very differently. These contradictions illustrate the esoteric nature of quantum mechanics, the study of nature at its smallest scale (the atomic and subatomic). No, wait, come back. I promise we are not going down that rabbit hole. While this duality concept is profoundly complicated and incredibly difficult to visualize, we will try to keep this as simple as possible just to give you an idea.
Light exhibits particle-like properties, consisting of a stream of discrete packets of energy, called photons. These are massless particles without a defined shape but can be individually registered and counted by sensitive detectors. They move in straight lines like tiny “bullets” of light. Photons exhibit particle-like behavior such as particle collisions, the ability to be absorbed or emitted by atoms, the photoelectric effect.
Light also exhibits wave-like properties, acting as a continuous oscillating wave of electric and magnetic fields that have a wavelength, amplitude and frequency. It exhibits wave-like behavior such as refraction, polarization, diffraction and interference. Everything here that we haven’t covered in previous sections will be covered in the sections ahead.
If it still seems confusing, don’t get discouraged because even scientists struggle with this part. This is the one aspect of light that is not going to be easily understood without diving into the deeply complex intricacies of quantum mechanics. Just understand that this dual nature should not be interpreted as light being sometimes a wave and sometimes a particle. Rather, it should be interpreted as a third thing altogether that has some things in common with both waves and particles. Here’s another way to look at: If you see a train, you might say it has “horse-house duality” in that a horse travels around taking you places like a train does and a house is a structure you can sit inside of just like you can in a train. Despite these commonalities, a train is not a horse, nor is it a house. It is a train.
HOW LIGHT MOVES AND INTERACTS WITH MATTER
BASICS
When light is emitted by a source, it is radiated outward in every possible direction in the form of oscillating wavefronts, much like ripples spreading across the surface of a pond, except here it appears more as expanding spheres of ripples with the light source at the center. Each individual wave will move in its respective direction forever until its path is interrupted in some way.
The speed of light, as was determined by James Clerk Maxwell in the 1860’s, moves through the vacuum of space at a constant 300,000 km/s (186,000 mi/s). Astronomers often use the speed of light to measure the vast distances of the cosmos. A light year is a unit representing the distance light travels in a year which is about 9.5 trillion kilometers (5.8 trillion miles). The speed of light is often denoted by the letter “c” in equations.
Light intensity decreases with the square of the distance from the source via the inverse square law. This is because every light ray is emitted outward at slightly different angles. The further away they get, the more their differing angles spread them apart across a larger and larger area. Therefore, a receiver of light will encounter less of the light, the further away it is.
If you double the distance that light travels, the intensity becomes 2 x 2 = 4 times dimmer (or 1/4 its initial intensity). If light travels 10 times further, the intensity becomes 10 x 10 = 100 times dimmer (or 1/100 its initial intensity). If this sounds familiar, it’s because this is how gravity weakens with distance too.
As the expanding spheres of wavefronts cross vast distances, they gradually start to resemble flat, planar wavefronts. Granted, they are always still curved but seem to flatten as they spread more and more. The curve eventually becomes negligible and the successive wavefronts appear parallel to each other.
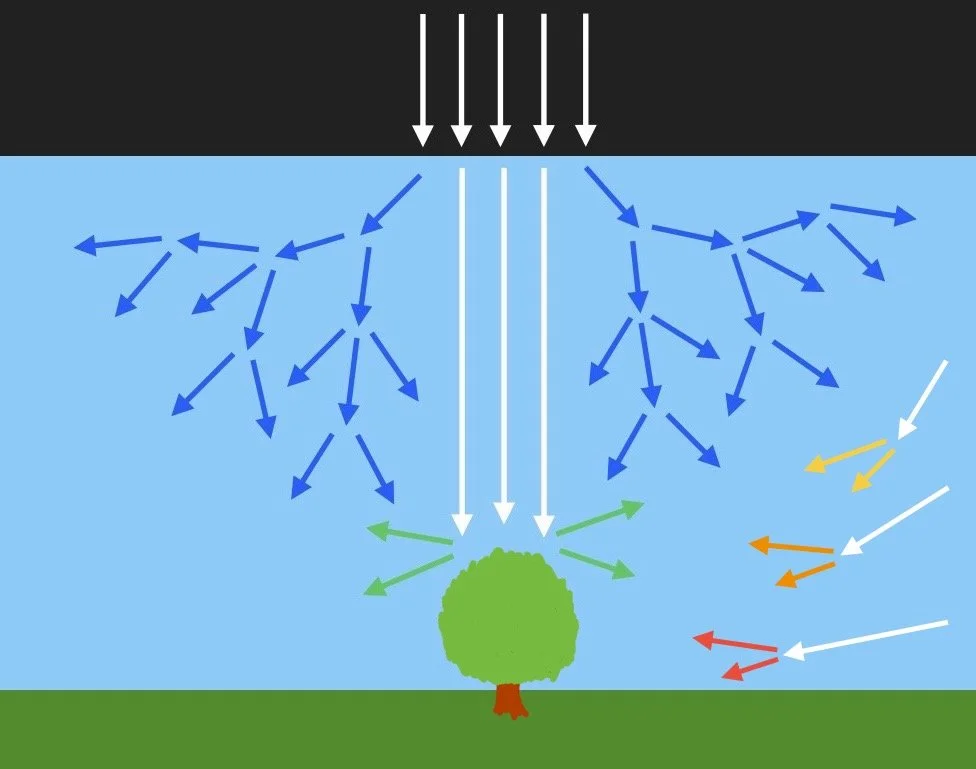
When light does make contact with an object like a planet, a cloud of gas or some other medium, there are different ways it can react depending on the shape, density and composition of the object. The interactions we will cover are:
The human eye
reflection
absorption
transmission
refraction
dispersion
scattering
polarization
diffraction
interference
photoelectric effect
THE HUMAN EYE
The most important light to us is the light we can see and the only light we can see is visible light that goes directly into our eyes.
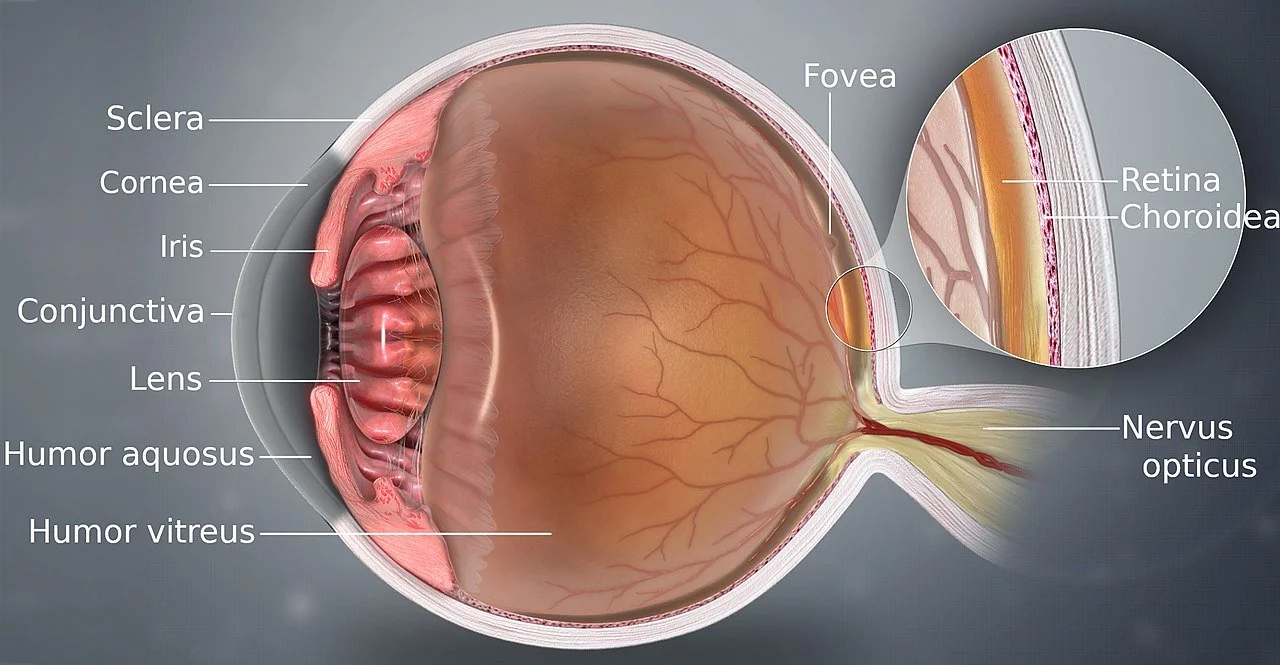
Light passes through the cornea, which is a transparent, dome-shaped structure that covers the front of our eye. The cornea helps focus the light via refraction into our pupils, which is the opening in the center of the iris, which is the colored part of the eye. This may sound like how a refractor telescope works because it is. The convex objective lens at the front of a refractor telescope focuses that light into a point.
The iris controls the size of the pupil by contracting and dilating, which controls how much light is able to pass through at once. Behind the pupil is the lens, which is another transparent, flexible structure that focuses the light coming in from the pupil towards the retina at the back of the eye.
The retina is a thin, light-sensitive layer of tissue lining the back of the eye. It contains two types of light-sensitive cells called rods and cones. Rods are responsible for distinguishing light and dark while cones are responsible for distinguishing colors. The retina’s purpose is to convert light that enters the eye into electrical signals that are sent to the brain via the optic nerves. The brain then processes and interprets these electrical signals and the end result is our visual perception.
The human eye is a complex and crucial tool for living our lives, but it is very limited in terms of the kind of light it can see. The very narrow range of the electromagnetic spectrum called visible light (from violet at 400 nanometers to red at 700 nanometers) covers all the light that the human eye is capable of processing. Any light outside of that range is more or less invisible to us and we must use technology to detect it.
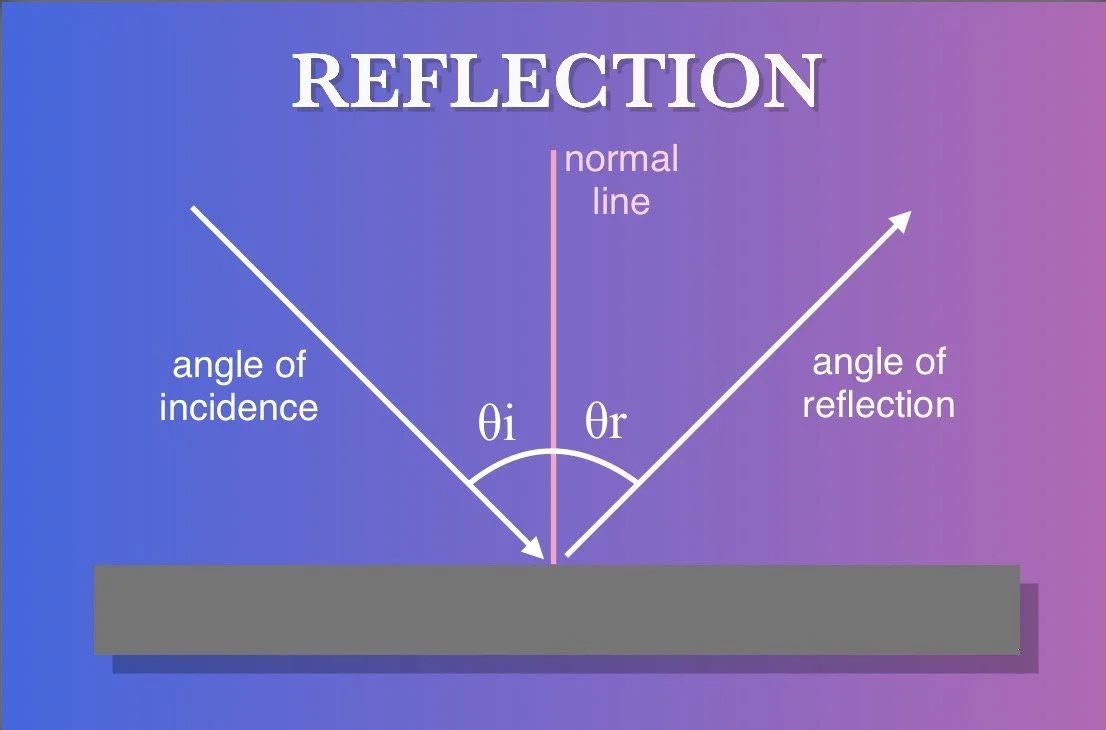
REFLECTION
When light strikes the surface of an object, it can be reflected. The angle of reflection (θr) depends on the angle of incidence (θi). This means that the light will bounce off the surface at the same angle it hit the surface, just in the opposite direction. The angle of incidence is measured between the path of light and the normal line (a line perpendicular to the surface at the point of incidence). For example, if light hits a flat surface at a 30° angle with respect to the normal, it will also be reflected at a 30° angle with respect to the normal. Alternatively, if light hits a flat surface head-on at a 90° angle with respect to the normal, it will be reflected back in the same direction it came from.
Whether or not light is reflected off an object’s surface depends on the object’s reflectivity. This is usually expressed as a percentage where 100% means a surface reflects all light that makes contact with it and 0% means a surface absorbs all light. Most mirrors have a reflectivity in the 90-95% range. Reflectivity depends on a number of factors including the object’s composition, surface roughness, the angle of incidence and the wavelength of the incoming light.
Reflectivity is crucial as the only way we are able to see the objects and environments around us is if light is first reflected off those objects and environments and then directly enter our eyes (unless, of course, the object itself is a light source).
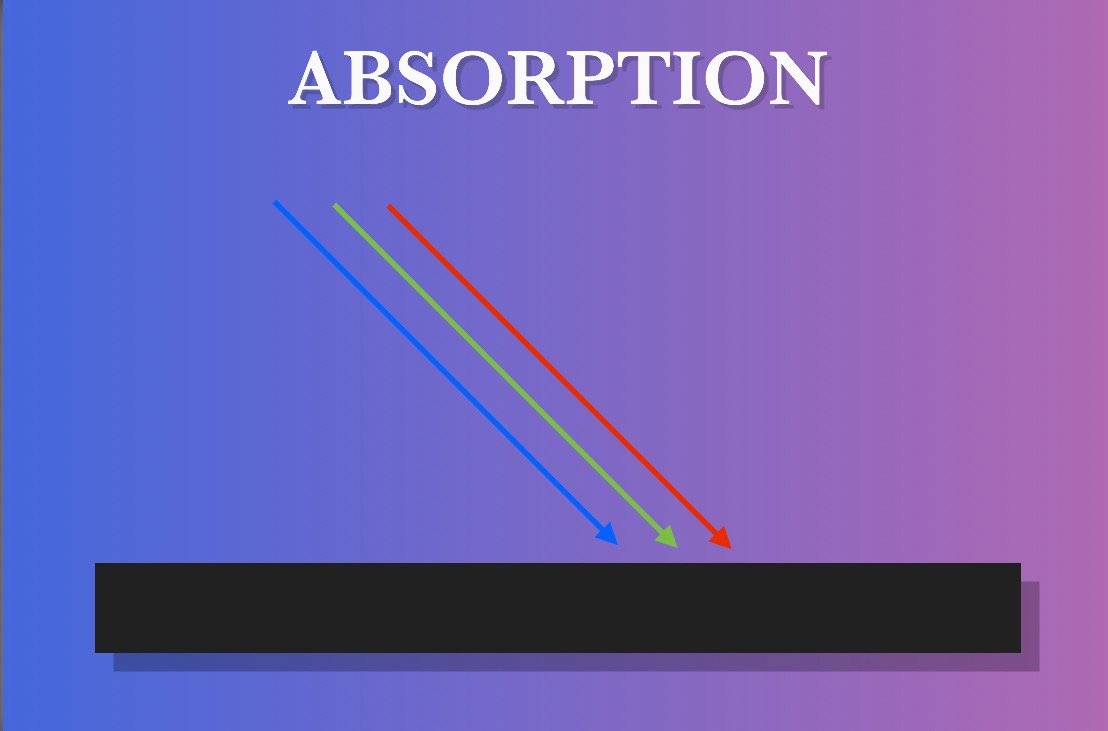
ABSORPTION
No object has 100% reflectivity. Even the most complex, well-crafted mirrors can only reflect up to 99.999% of the light it receives. Any light that is not reflected, transmitted or otherwise allowed to escape the object is absorbed by the object. When light is absorbed, the object becomes imbued with energy. The electrons of the object’s atoms become excited and move to a higher energy level before eventually releasing the energy as heat. This is why darker-colored objects feel hotter than brighter-colored objects.
Note: This is not to be confused with atoms emitting light from relaxing electrons as seen in luminescent objects.
What light gets absorbed or reflected depends on the wavelengths of the light versus the element of the atom. Remember that electrons can only be moved to a higher energy level if they absorb a sufficient amount of energy. Some energy levels require more energy than others, depending on the element of the atom. So, if the energy of the light’s wavelength matches the energy needed to level up the electron, the light is absorbed. If it is just shy of the amount needed, the light is reflected or transmitted.
The absorption of some wavelengths of light combined with the reflection of other wavelengths is what gives an object its color. For example, a leaf on a tree appears green to us because it absorbs all the Sun’s wavelengths of white light except for green. The green wavelengths are instead reflected outward and some of it enters our eye and we perceive the leaf as that color. If an object absorbs all wavelengths of light, that object will appear black. However, just as there are no real-world examples of a perfect reflector, we also don’t have a perfect absorber. The closest thing we have to the latter are black holes.
TRANSMISSION
Some materials can allow light to pass through it instead of being reflected or absorbed. This is called transmission and the ability of a material to allow this is called transparency.
Transparent material that allows the transmission of light tends to have:
Simple atoms with a dense, highly ordered, uniform structure allowing for light transmission without disruption (silicon, carbon, hydrogen, oxygen, nitrogen, some rare earth elements).
Tightly bound electrons with a large energy threshold for excitation, meaning they are less likely to absorb light (see absorption).
A low refractive index that prevents the bending of light (see refraction).
Low polarizability which will not prevent light from passing through based on the orientation of its oscillating waves.
Minimal impurities, irregularities or defects that prevent internal scattering that turns the object hazy or opaque (see scattering).
Some materials are more transparent than others.
Materials with a high level of transparency will transmit most or almost all light that interacts with it. Remember that just as no known material is a perfect reflector or perfect absorber, so too is no known material perfectly transparent.
Some natural examples of transparent material: Diamonds (transparent solid), pure water (transparent liquid), air (transparent gas)
Some manufactured examples of transparent material: glass (for windows and lenses), synthetic plastics (such as acrylic)
Materials with a more moderate level of transparency, called semi-transparent or translucent, will only allow some light to pass through it while the rest is reflected, absorbed or otherwise diverted away. Some materials may only allow through certain kinds of light such as certain colors or light with certain wave orientation (polarization).
Some natural examples of semi-transparent material: seawater (due to salt and particulates), quartz crystals, marine organisms (jellyfish)
Some manufactured examples of semi-transparent material: telescope filters, sunglasses, stained or frosted glass, etc.
Objects that transmit no light are considered opaque.
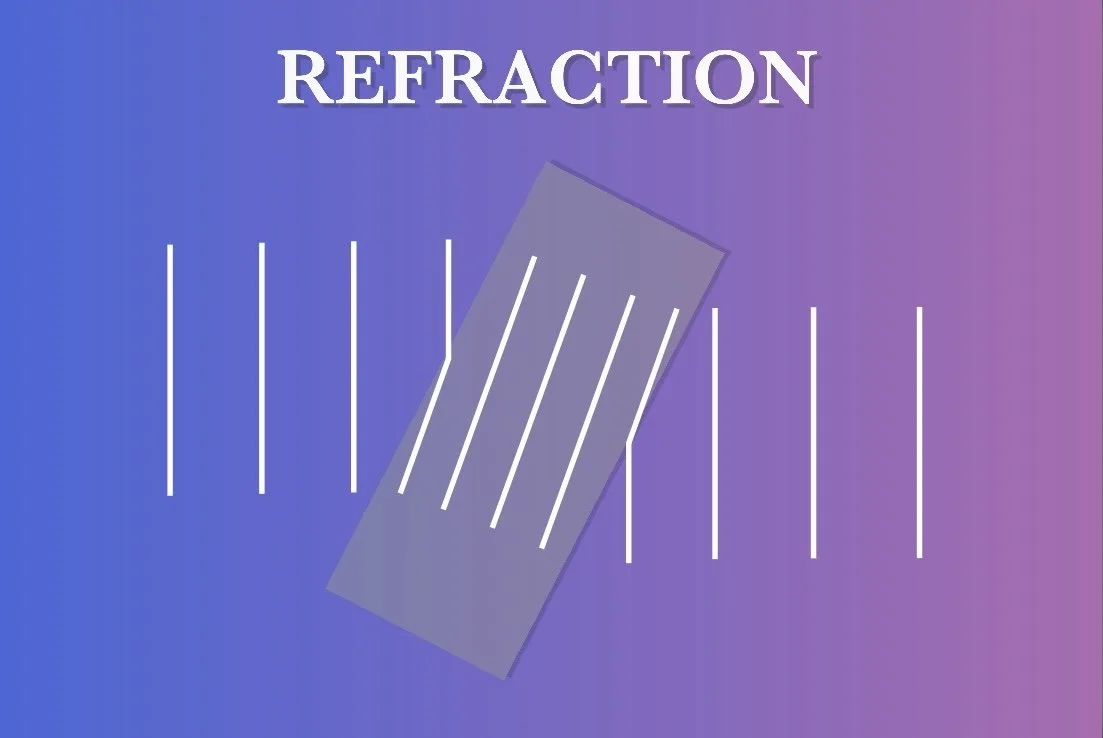
REFRACTION
When light transmits at an angle from one medium to another, such as from air to water or glass, the light is bent or refracted. This is because the speed of light changes as it crosses the boundary between the two mediums. Why? Different mediums have a different refractive index. This property quantifies how much the speed of light is reduced when traveling through the medium compared to the speed of light in a vacuum. Two different mediums will have two different refractive indices and will allow light to move at different speeds. Moving from a lower refractive index (like air) to a higher refractive index (like water or glass) will result in the light slowing down. Likewise, moving from a higher refractive index to a lower refractive index will result in the light speeding up.
Why does the change in the speed of light change the direction of the light?
Imagine wavefronts of light transmitting through a slab of glass at an angle (pictured). As the wave enters the glass, a portion of it will be in the glass while the other is still in the air. Light travels more slowly in glass, so the wavefronts are closer together in glass than they are in air. The only way the wavefront can remain continuous is if the wavefront tilts in the glass. Which means the beam as a whole bends down. According to Snell’s law, the bigger the difference between the two mediums’ refractive indices, the more the light is refracted.
Again, this bending only happens when the surface between two mediums is at an angle to the beam of light. If the surface was perpendicular to the light beam, the light would simply pass straight through, albeit at a different speed. This is why if you look through a glass of water only the edges appear distorted, but the middle appears mostly normal.
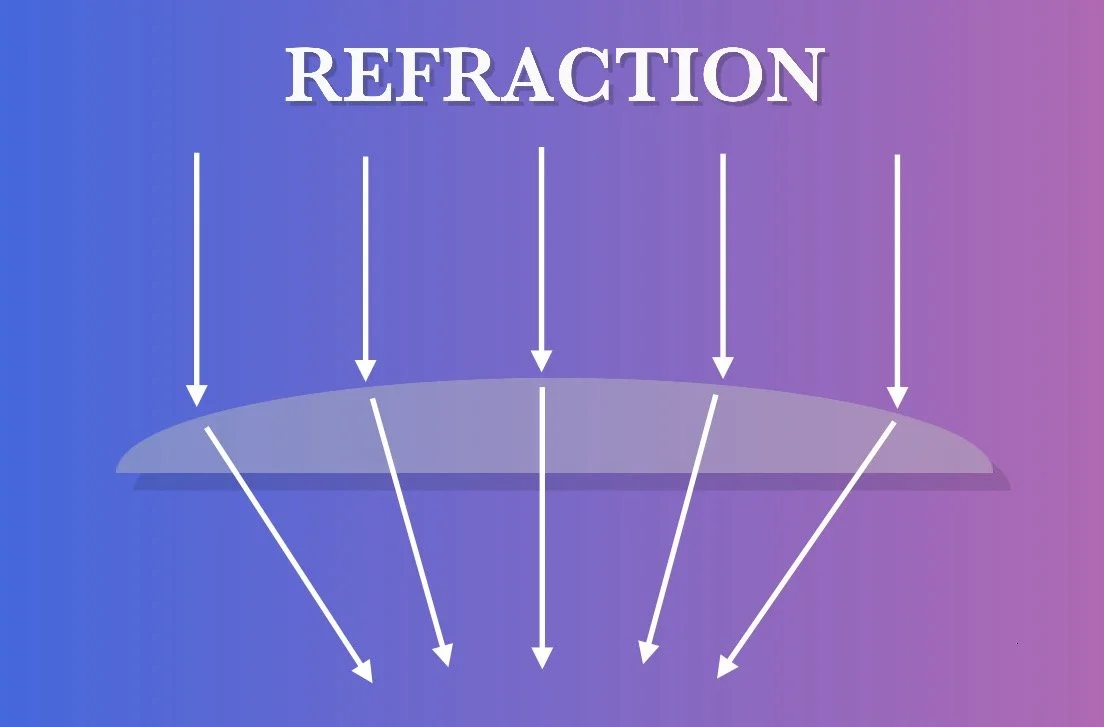
One of the greatest innovations in the history of astronomy is the invention of lenses, specially crafted pieces of glass which refract light in a controlled manner. A convex lens, like the one shown in this graphic, is curved outward in a way that it refracts light inward with the outer edge refracting light at the greatest angle and the center allowing light to pass straight through. This focuses all the incoming light into a small point. Telescopes that use these convex lenses are called refractor telescopes because they use the refraction to funnel light into the eyepiece so that the image can be magnified.
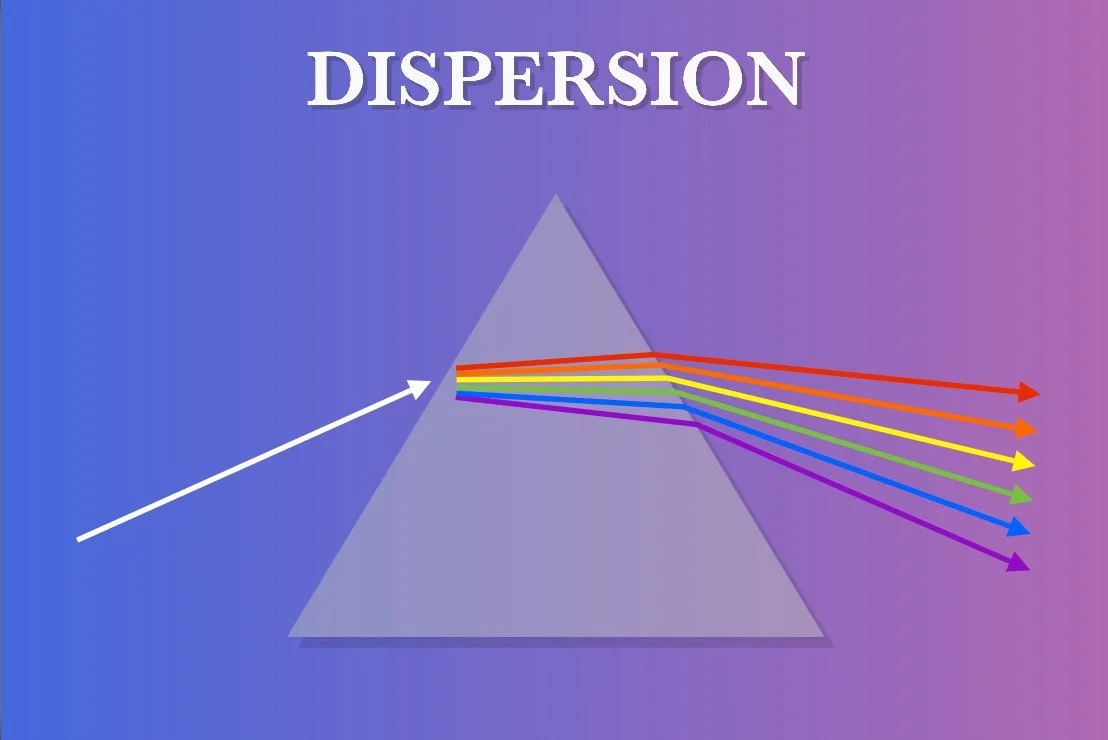
DISPERSION
As you know by now, when light is passed through from one medium to another, the light is refracted or bent. You should also know that white light is a combination of all the different wavelengths, or colors.
When white light is refracted in this way, there is an interesting phenomenon in which the different wavelengths that make up the white light are refracted at different angles. Red wavelengths are refracted at the least angle while violet wavelengths are refracted at the greatest angle. These different angles allow the wavelengths to separate from each other and spread outward. This phenomenon is called dispersion.
This effect is most famously demonstrated using an object called a prism. These are pieces of glass with a high refractive index and crafted into a triangular shape. This creates an exaggerated refraction and dispersion effect. Prisms can be used in other forms as part of a spectroscope or pair of binoculars.
Dispersion happens in nature as well. In the presence of mist, such as following a rainstorm or when standing near a waterfall, many small droplets of water vapor are floating around the air. As white sunlight passes from the air through the water droplets, it is refracted and dispersed into its separate colors. From inside the water droplet, the light is then reflected off the back side and finally exits the droplet. This disperses the light even more as it changes mediums again. This dispersed light being reflected back at us is what creates rainbows. This will appear as a colorful arc in the sky in which the colors are arranged in a particular order: (from top to bottom) red, orange, yellow, green, blue, violet. A similar phenomenon is the halo of light that occasionally appears around the moon as the moon’s light is refracted off high-altitude ice crystals in Earth’s atmosphere.
Dispersion is also why you can see the color spectrum in the reflection of water or in soap bubbles.
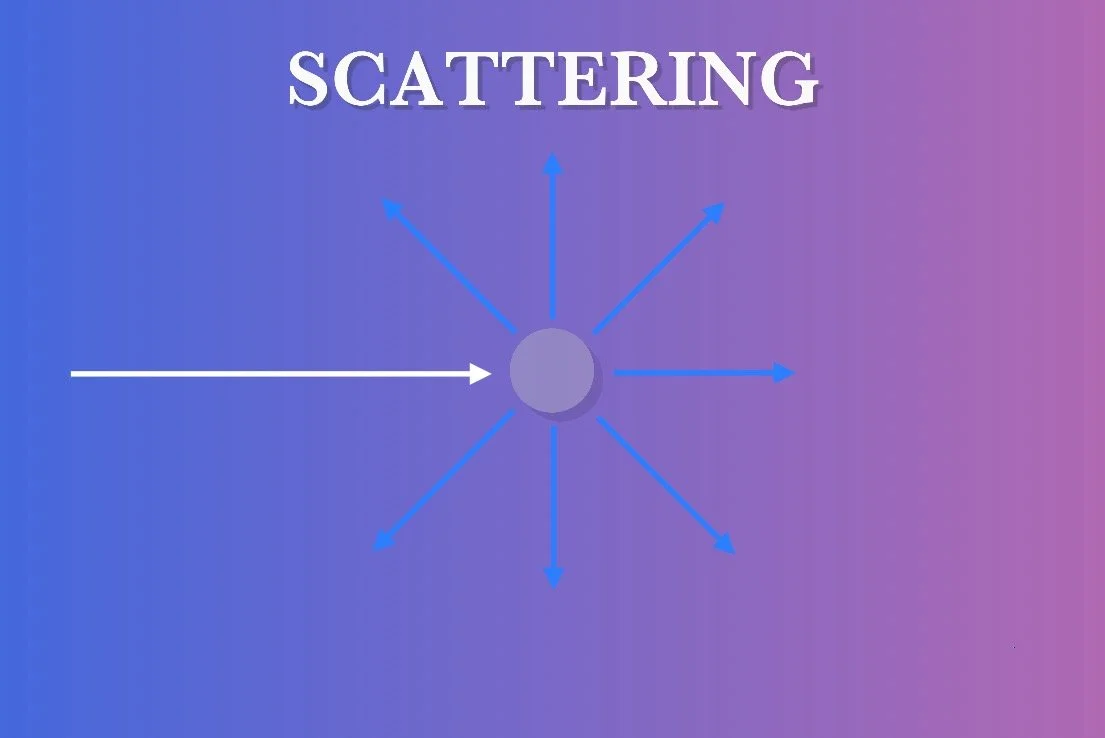
SCATTERING
Light coming into contact with small particles or molecules in the atmosphere, as opposed to large mediums, can cause the light to redirect its component wavelengths in separate directions. This is called scattering. This is distinct dispersion wherein light is refracted upon passing through material.
There are different types of scattering that occur under different circumstances but the most common are Rayleigh scattering and Mie scattering.
Rayleigh scattering occurs when the scattering particles are much smaller than the wavelength of light. Shorter, blue wavelengths are higher energy and are thus scattered much more efficiently by the smaller particles than longer, red wavelengths. A prolific example of this is the air molecules in Earth’s atmosphere (molecular nitrogen and oxygen). When the white light from the Sun enters Earth’s atmosphere, the blue wavelengths are scattered much more and thus the sky appears blue in the daytime. As the Sun descends toward the horizon, the light is reaching through the atmosphere at a lower and lower angle, scattering more and more of the blue light away. By the time the sunlight reaches you, most or all of the blue has been scattered away leaving only red light remaining.
Mie scattering occurs more predominantly when the scattering particles are comparable in size to the wavelength of light or larger. This would be instances in which the air is littered with thicker particulate matter such as water droplets, dust or smoke. This primarily occurs in the lower atmosphere where larger particles tend to settle. While Rayleigh scattering will scatter the light in every direction, Mie scattering will scatter light more heavily in the direction of the incidence (the direction the light was initially moving). Mie scattering is not strongly wavelength dependent and will scatter wavelengths more evenly across the spectrum. This is where we get the pale hues of clouds, mist and fog. It also produces the white glare around the Sun and the haloes we see around the Sun and Moon when the air is thick with ice crystals.
Scattering happens to non-visible light as well. With ultraviolet wavelengths, the effects of Rayleigh scattering are even more pronounced than blue visible light while infrared is scattered less than red visible light. X-rays and gamma rays can experience something called Compton scattering in which X-ray or gamma ray photons collide with charged particles, typically free or loosely-bound electrons. The collision transfers some of the photon's energy to the electron, increasing the photon's wavelength and deflecting its path. The greater the angle of deflection, the more energy is transferred. Meanwhile, the recoiled electron is likely to be ejected from its atom.
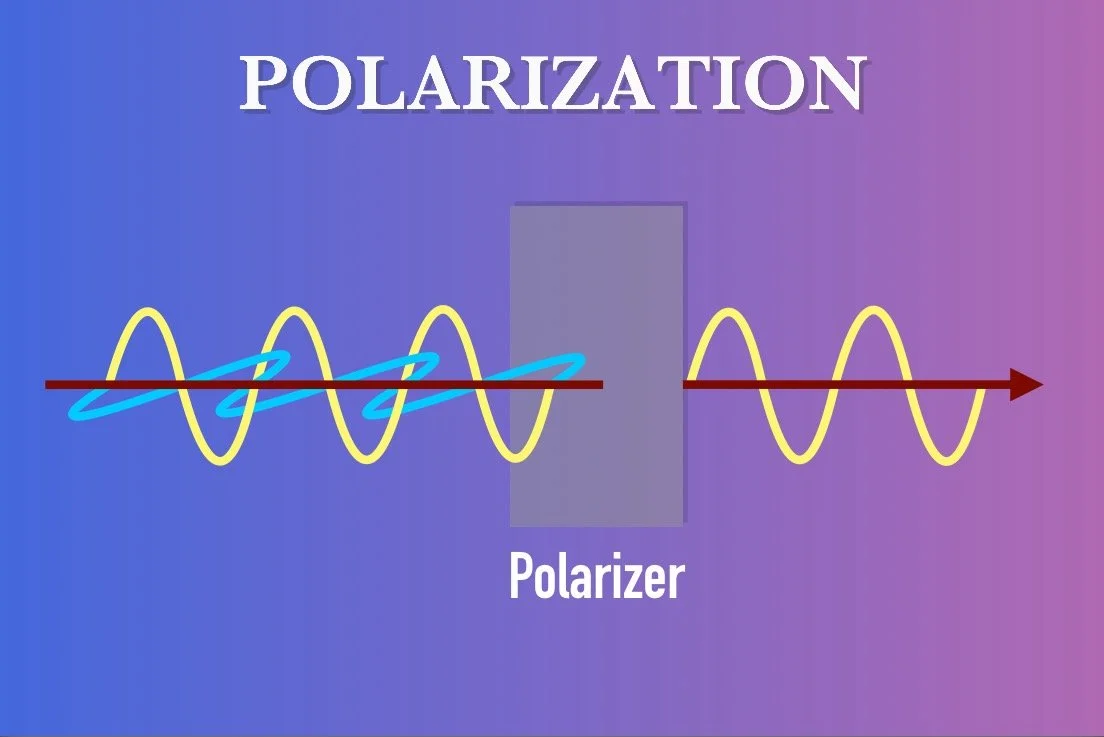
POLARIZATION
When light travels, the oscillation of the waves is perpendicular to the direction of travel. However, that doesn’t tell you the orientation of the wave, which is to say the direction that the oscillations are moving towards and from. This orientation is known as the light’s polarization. Light can be polarized in a number of different ways. It could be polarized linearly, meaning the wavelengths oscillate on a single continuous plane. It could also be polarized circularly, in which the oscillation of the wavelengths rotate (either clockwise or counterclockwise) with the direction of travel acting as the axis of rotation. Elliptical polarization describes a combination of the first two. If light is unpolarized, then the waves oscillate in random directions like we see with sunlight.
These behaviors can be manipulated using a number of techniques:
Polarizing filters such as those used in telescope filters, sunglasses and cameras selectively transmit and block light based on its polarization.
Liquid crystal displays (LCDs) rely on the ability to control the polarization of light to create images.
Optical communications can use polarization to transmit information through fiber optics cables.
Measuring polarization can be used to learn about spectroscopy
Satellites can measure polarization to study atmospheric, oceanic and land surface properties.
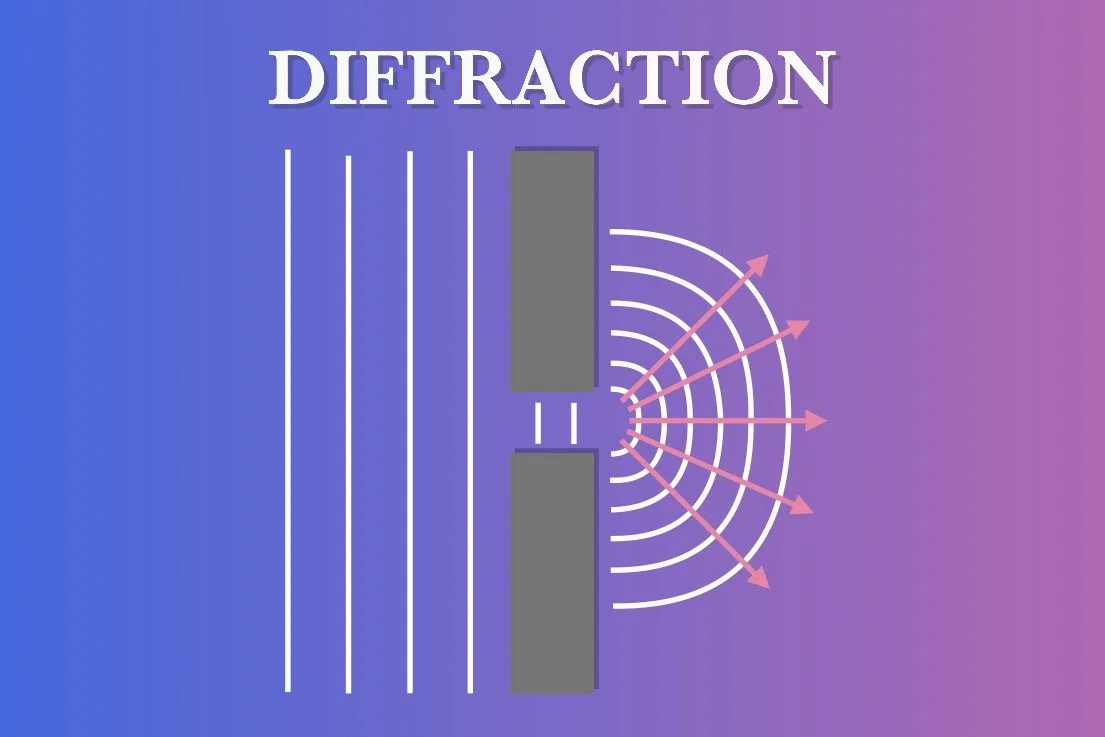
DIFFRACTION
Diffraction is a phenomenon where waves, such as light, bend and spread out when they encounter an obstacle or pass through a narrow opening. Imagine a wavefront of light approaching a wall with a slit cut into the middle. Only the light that passes through the slit can continue to the other side. When the light passes through, the narrow slit generates new waves that spread out in all directions as if the slit were a new source of light. This is called diffraction. The extent of diffraction depends on both the width of the slit and the wavelength of the light. If the slit is wide compared to the wavelength, the light will travel in a straight line with minimal spreading. Conversely, a narrow slit allows for more pronounced diffraction, spreading in all directions. The wavelength also plays a critical role; longer wavelengths, such as red light, will diffract more than shorter wavelengths, such as blue light, resulting in a phenomenon called "color separation."
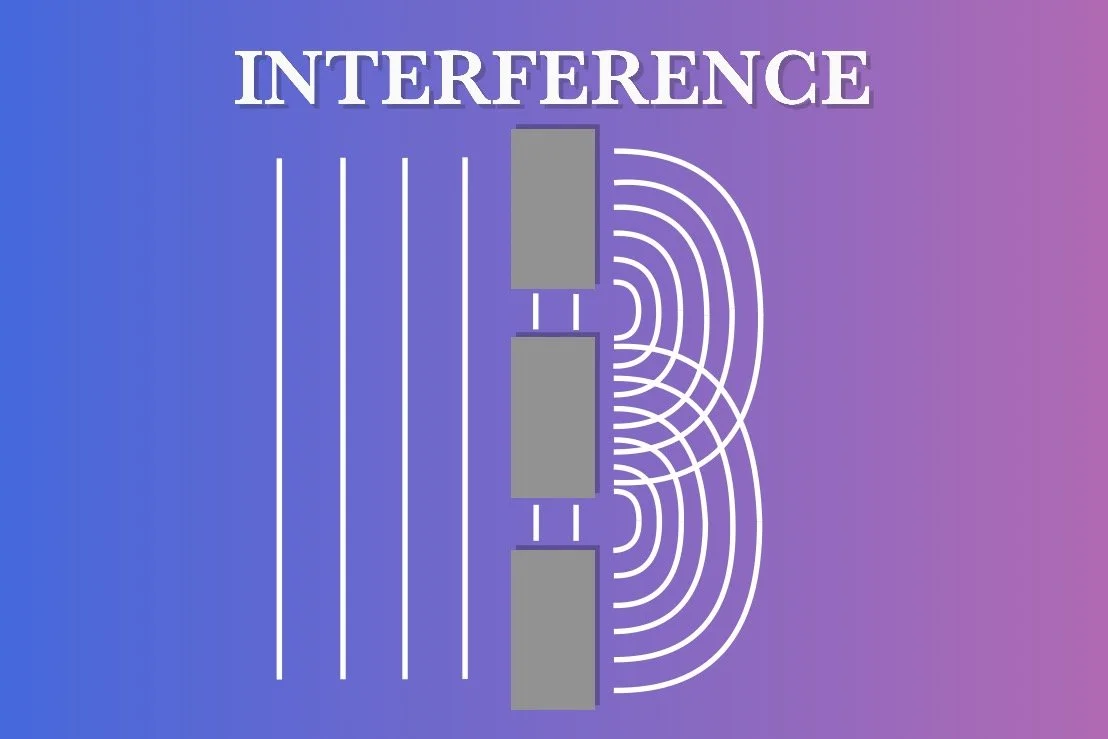
INTERFERENCE
If there are multiple narrow slits in the wall, each slit will produce its own set of wavefronts that can overlap with each other. This results in interference. Depending on whether the waves are in phase (their peaks and troughs align) or out of phase (the peaks and troughs oppose), they can either amplify each other (constructive interference) or cancel each other out (destructive interference). As the diffracted wavefronts travel towards another wall, they create an interference pattern of alternating dark and light regions. This pattern results from the varying degrees of constructive and destructive interference between the overlapping wavefronts. This phenomenon was famously demonstrated in Thomas Young’s double-slit experiment in 1801.
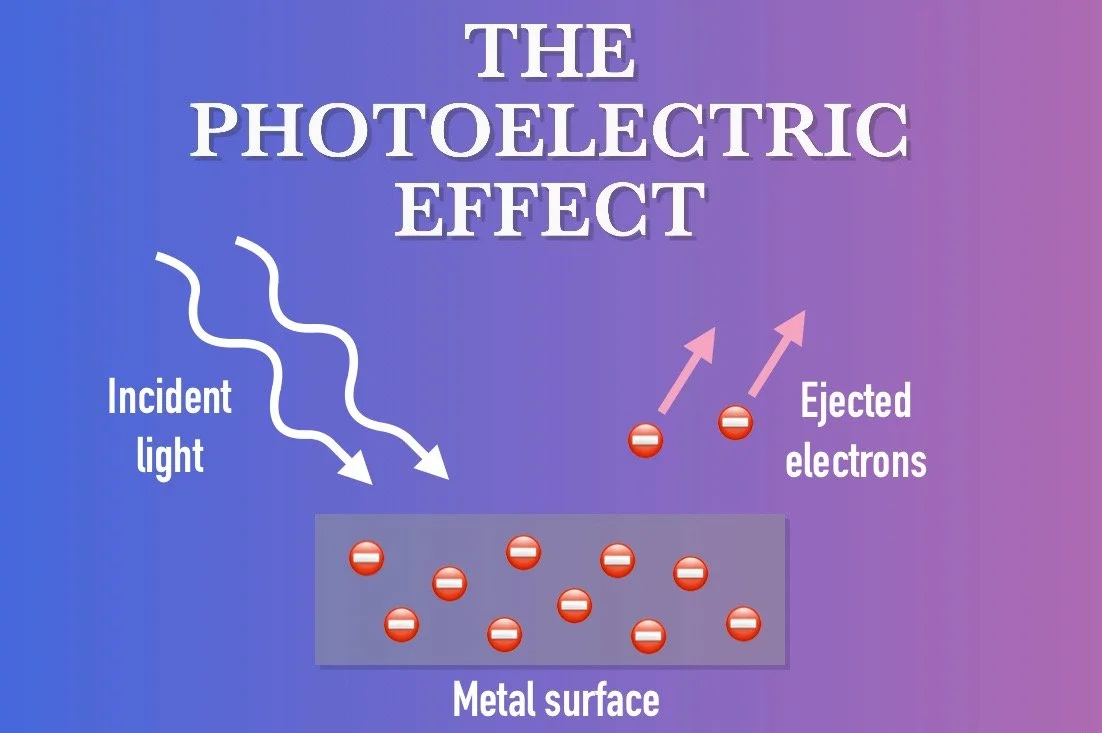
THE PHOTOELECTRIC EFFECT
In 1905, the same year he released his theory of special relativity, Albert Einstein worked to unravel the mysteries of the photoelectric effect. Ironically, it is not Einstein’s famous relativity papers that won him the Nobel prize. It was the paper he wrote on this phenomenon that earned him the honor in 1921.
The photoelectric effect is a phenomenon in which if you shine a light onto a metal surface in the right way, electrons can be emitted and ejected outward. In other words, the energy of the light, aka the electromagnetic radiation, collides with the electrons that are loosely bound to the metal, and they are knocked loose. The interesting thing is that the energy with which the electrons are ejected is not determined by the intensity of the light but by the light’s color. The intensity of the light instead determines the number of electrons that are ejected. This is a strange paradox between the color and intensity of light; one that Einstein set to work out.
Einstein explained this phenomenon by returning to an old idea that can actually be traced back to Sir Isaac Newton. Newton’s idea was that light is not just some ethereal medium as people generally observe but a stream of particles, aka photons. In Einstein’s version, light is described as being both a wave and a stream of particles, simultaneously. How does this resolve the problem of the photoelectric effect?
When light shines on a metallic substance, individual photons within the beam of light collide with individual electrons, one-to-one. The energy of the photon is dependent on the frequency of the light. As you may know, the color of light is determined by how long or short its wavelengths are. Red light has very long, less energetic wavelengths while blue and violet have very short, highly energetic wavelengths. The higher the energy of the wavelengths, the more energy the photon has to eject the electron faster. The brighter the light, the more photons there are to collide with more electrons.
This concept of light as a series of photons that travel in a wave-like formation, was foundational to the beginnings of quantum mechanics.
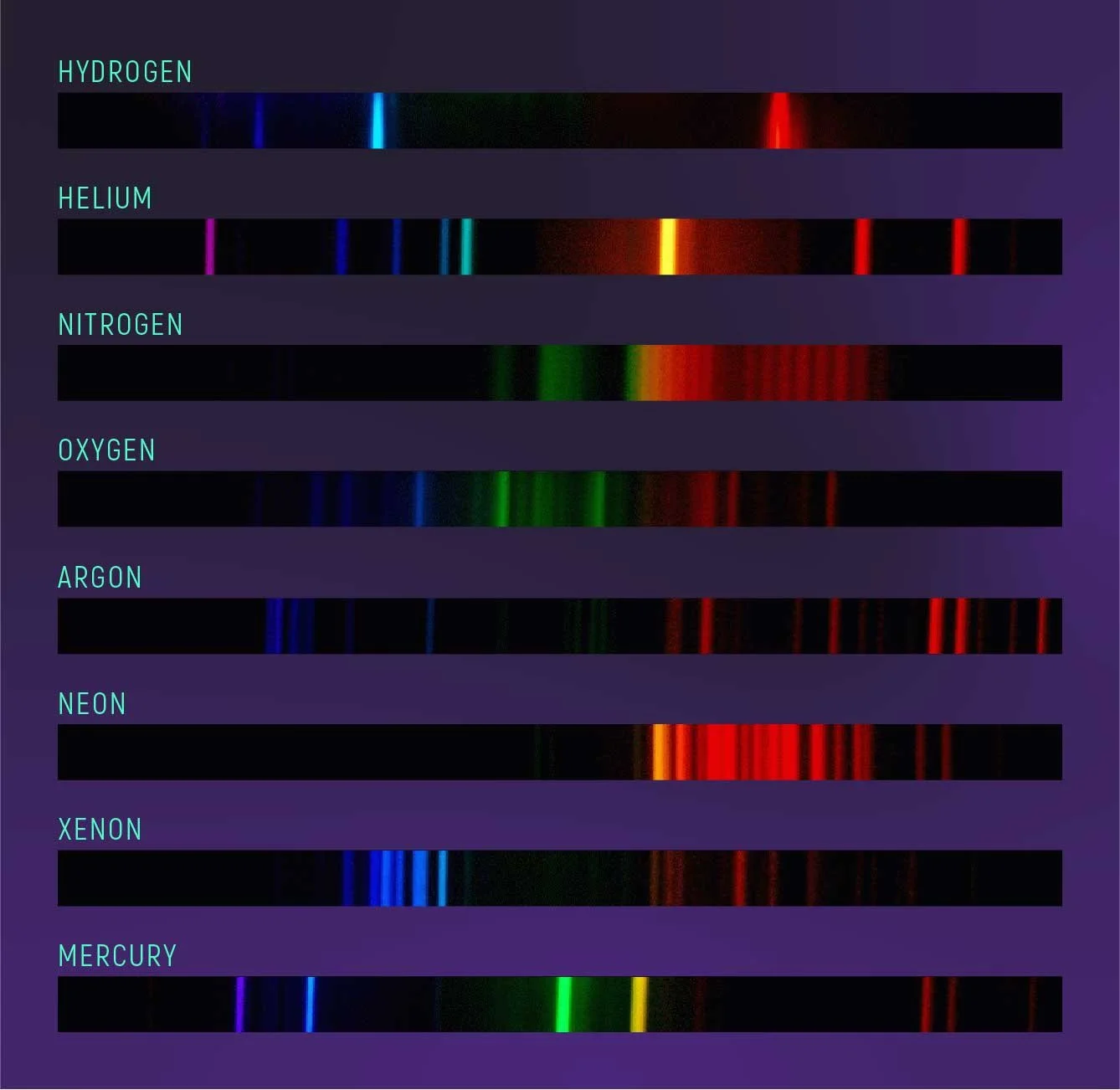
SPECTROSCOPY
Atoms come in many different elements such as hydrogen, oxygen, carbon, etc. The element is determined by the number of protons that are in the atom’s nucleus. For example, all carbon atoms have six protons.
Returning to our previous electron stair analogy used in the luminescence section, each atom of different elements will have a different, unique set of stairs. The steps on each atom’s stairs can vary in height with one step being taller than the one above or below it and other steps being very short. The taller the step, the more energy the electron must absorb to get to the next step. The result of these differences is that when light reflects off of some kind of matter, depending on what element it is, it will reflect light of different wavelengths (or colors) while absorbing others.
In spectroscopy, by analyzing the wavelengths of light coming from an object, even a very distant one, scientists can actually determine what element that object is made of. This analysis is made possible by the use of spectrometers which can separate the numerous wavelengths from incoming light allowing scientists to study what is called the emission lines. Light reflected from a particular element will give off a specific recognizable set of emission spectra.
Spectrometers can even be used on telescopes which allow us to analyze the light from distant stars and exoplanets. This is how astronomers are able to know what is going on inside and around these objects even though they may be millions of light years away. Spectroscopy can also tell us how hot a star is or tell us about a star’s metallicity which in turn helps us determine what generation it belongs to. We can figure out whether an exoplanet has water or carbon dioxide in its atmosphere. We can even tell whether a distant object is moving towards or away from us and how fast.
THE DOPPLER EFFECT
Have you ever noticed when you are standing on the side of the road, the pitch of a car’s engine seems to get higher as it approaches you and then gets lower as the car moves away from you? Why does this happen? As the car moves, the sound waves in front of the car are being compressed, creating a shorter wavelength and thus a higher pitch. Meanwhile, the sound waves behind the car are being stretched out, creating a longer wavelength and thus a lower pitch. This phenomenon is known as the Doppler effect. We commonly associate this with sound, but it also happens with light (remember that they are both waves)!
If an object is moving towards you, the wavelength of the light that is being emitted or reflected from that object will also be compressed (or shortened). If an object is moving away from you, the wavelength will be stretched (or lengthened). With sound, this change in wavelength affects the sound’s pitch to be higher or lower. How does this affect light?
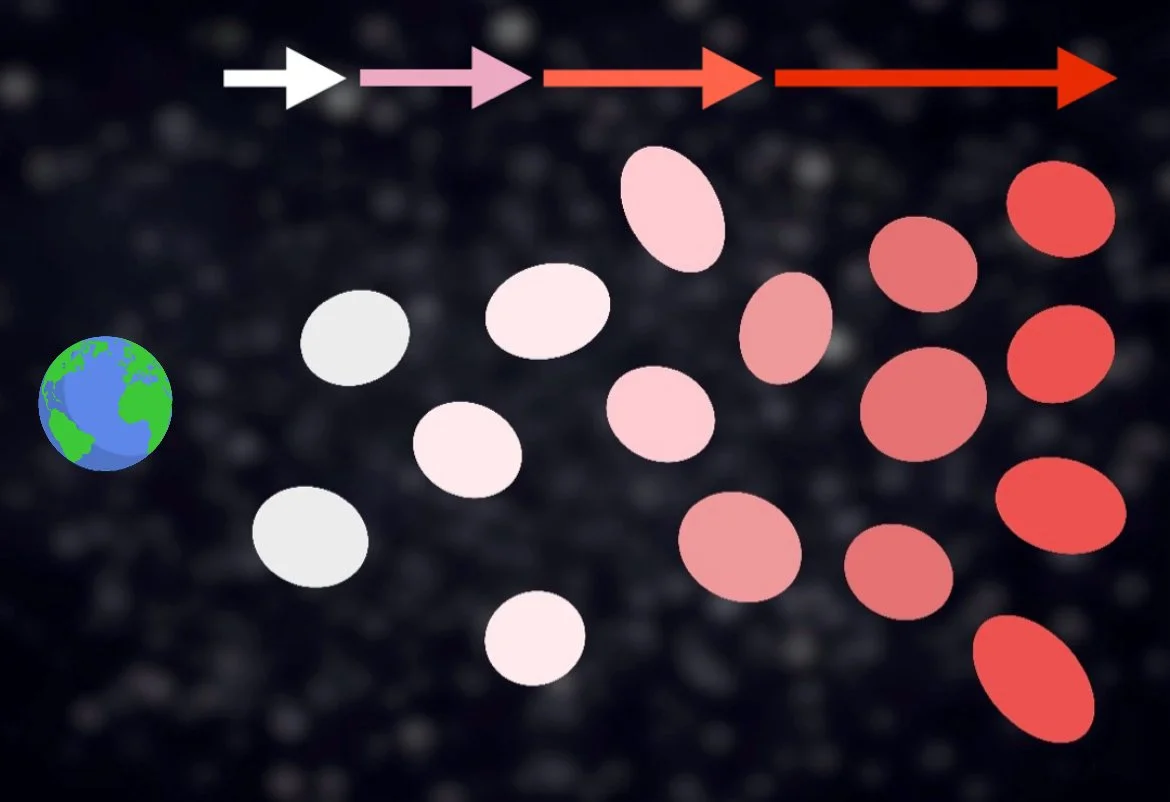
It changes the light’s color!
As we learned in the visible light section, the wavelength of light corresponds with different colors. The longest wavelengths of visible light appear red while the shortest appear blue and violet. As we learned in the previous section on spectroscopy, we can see recognizable patterns of emission and absorption lines across the light spectrum of an object based off the elements present within it. So, when an object moves toward you, the various spectral lines we find in that object will shift as a whole towards the red end of the spectrum. Meanwhile, if the object is moving away from you, we see those same spectral lines shift towards the blue end of the spectrum instead. This is called redshifting and blueshifting, respectively.
While violet is technically the shortest wavelength of visible light, astronomers who first established these terms felt blue was a more intuitive color to pick. Blue is more broadly present on the color spectrum, and the human eye is more sensitive to blue than violet, Earth’s sky appears blue in the daylight, the hottest stars appear blue, etc.
Try not to take “redder” vs “bluer” adjectives too literally. If a car is driving away from you or towards you, don’t expect to see the car actually turn red or blue. It’s just that the spectral lines that the car would normally present when stationary is shifted ever so slightly in one direction or the other (check the right side of the accompanying image). In fact, you’re not likely to ever notice it happening like you do with sound. Why not? It has to do with speed.
Sound moves really fast through our atmosphere compared to most things we encounter in our daily lives. With things like temperature, humidity and altitude acting as varying factors, the speed of sound on average is 1,235 km/h (767 mph). However, light moves much, much faster. In a vacuum, light travels about 300,000 km/second (186,000 mi/second). This is approximately 875,000 times the speed of sound. It takes almost three seconds for a sound wave to travel 1 km while light can do 7.5 laps around the entire Earth in just one second. The speed of light can also be affected by external factors such as what medium it travels through, the type of light, the presence of gravitational fields, etc. but requires more extreme circumstances for us to perceive it.
To see the Doppler effect, you’ll need to observe objects moving incredibly fast across vast distances. The faster an object is moving, the more pronounced this effect will be. As it turns out, studying the cosmos will have you dealing with these types of speeds and distances regularly and the ability to measure Doppler shifts has transformed the field of astronomy.
The ability to detect a very far away celestial object’s Doppler shift allows us to learn some important things about it.
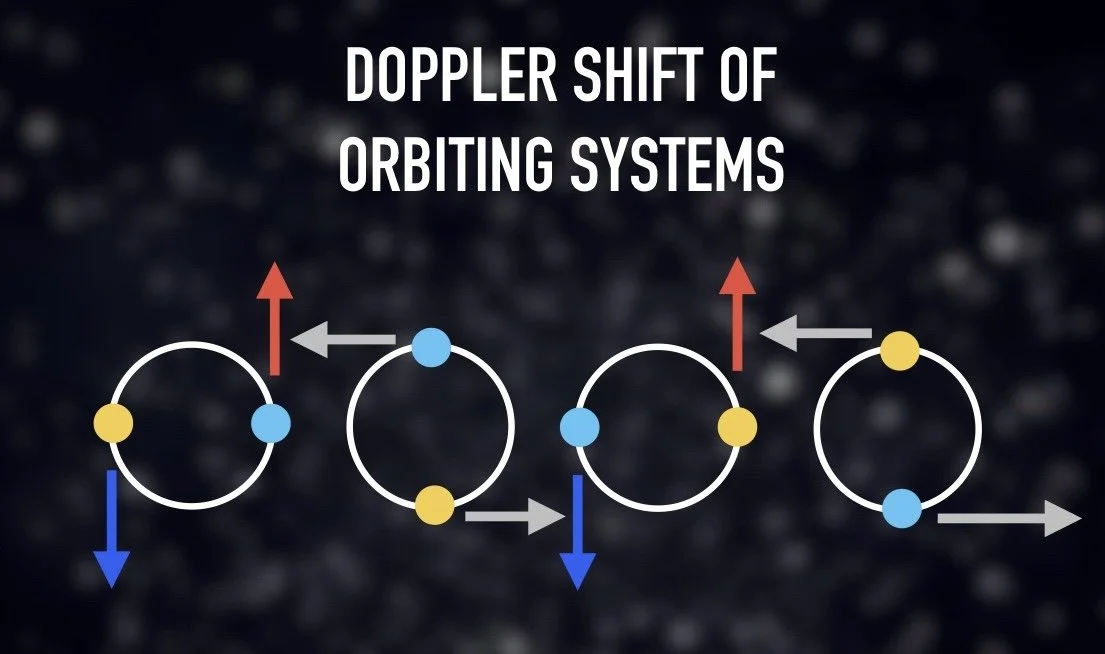
For example, we can determine what direction it is moving and how fast it is moving. If we are looking at a star that is significantly blueshifted, we know that means the star is moving towards us at a very fast speed. The star may often be moving diagonally relative to Earth. In this case, we must compare its Doppler shift (the speed it is moving towards or away from you) to its proper motion (its apparent movement across the sky over time).
We can also use the Doppler effect to identify orbiting systems, like binary stars. A lot of binary stars are so close to each other that we can’t rely on angular separation to detect them. Let’s assume a pair of stars in a binary orbit seen edge on. We would look for a cyclical pattern that goes between one side showing a blueshift while the other is redshifted and then there being no Doppler shift at all. This not only tells us that the stars are orbiting each other but tells us the direction of orbit.
We can even determine the speed and direction of rotation of an individual star and planet or galaxy using these same patterns. When analyzing the Doppler shift, we can compare one side of the object that is more redshifted with the other side that is more blueshifted, which reveals the direction and speed that it is rotating.
A limitation of using the Doppler effect to determine motion is if the orbiting system is face on to us and thus there is no motion towards or away from us. In this scenario, a very tight binary star system would be indistinguishable from a single star. Galactic rotation would be too slow to perceive its proper motion. Luckily, the majority of rotating or orbiting systems are neither edge on nor face on, but somewhere in between.
The Doppler effect has also been observed at the most extreme levels found in the universe. In 1929, American astronomer Edwin Hubble discovered something profound when he analyzed the Doppler shifts of really distant galaxies. He noticed that not only are galaxies moving away from us (aka they are redshifted) but that the further away they are, the faster away from us they are moving (the more redshifted they are). In fact, he calculated that the distance of a galaxy is directly proportional to the speed of its recession. This is known as Hubble’s Law and is one of the foundational pieces of evidence for the big bang theory and the expansion of the universe. It’s all thanks to the Doppler effect.
Light is the silent artist that paints the world around us with color and clarity. By deciphering the intricate tapestry of the cosmos, we can learn not only to see with our eyes but with our minds.
If you or anyone you know are looking for guidance on how light illuminates the mysteries of the universe, members of the Harford County Astronomical Society are here for you to provide our enthusiastic knowledge and experience.
Come find us at future events or fill out an outreach request form and ask for assistance and we will do what we can!